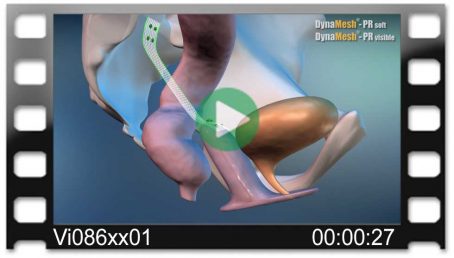
Rahim Ağzı Güdük Sarkması

PR
DynaMesh®-PR yumuşak ve DynaMesh®-PR görünür implantlar, apikal pelvik organ prolapsusuna yönelik cerrahi tedavinin bir parçası olarak köprüleme malzemesi olarak kullanılmak ve vajinal duvarların yumuşak dokusunu güçlendirmek üzere tasarlanmıştır.
Cerrahi teknik:
- Kolposakropeksi
tek taraflı
(eş zamanlı sistosel/rektosel)
Paylaş
The target patient group are fully-grown female patients with apical pelvic organ prolapse (of the uterus, vaginal or cervical stump).

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump and anterior mesh plasty for concomitant cystocele

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump and posterior mesh plasty for concomitant rectocele

Hysterosacropexy
- unilateral
- posterior cervical fixation

Hysterosacropexy
- unilateral
- posterior cervical fixation and posterior mesh plasty for concomitant rectocele
| DynaMesh®-PR soft |
04 cm x 23 cm | PV500423F1/F3/F5 |
| DynaMesh®-PR visible | 04 cm x 23 cm | PV700423F1 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
| Product | DynaMesh®-PR soft (1) DynaMesh®-PR visible (2) |
| Surgical Treatment | Apical Pelvic Organ Prolapse (Uterus / Vaginal Stump / Cervical Stump) |
| Surgical Approach | Minimally Invasive / Open |
| Surgical Technique | Sacropexy |
| Fixation | – Anterior longitudinal ligament: non-absorbable suture or tacks – Vaginal stump or cervix: interrupted non-absorbable suture (preferably) |
| Smooth Warp-Knitted Selvedges | |
| Defined Elasticity |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Effective Porosity | |
| Klinge’s Mesh Classification | Class 1a [102P, TR111] |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |